October 3, 2016 — The FDA is warning that homeopathic teething tablets and gels may pose a risk to infants and children.
There have been more reports of infants and children who experienced seizures after being given homeopathic teething tablets.
The FDA will be testing more products and updating the public with new information. In the meantime, the agency is asking consumers not to use the products and throw them away.
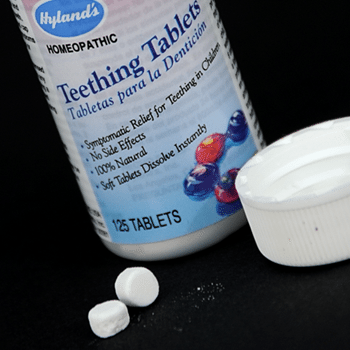
The FDA recalled Hyland’s Teething Tablets in October 2010 after receiving reports of children who had symptoms consistent with belladonna toxicity.
Belladonna, also known as “deadly nightshade,” is an extremely poisonous plant. It has been used since ancient times as a medicine, cosmetic, and in warfare. It has a mild anesthetic effect in small amounts.
The FDA found inconsistent amounts of belladonna in Hyland’s Teething Tablets. The agency also received reports of children consuming more tablets than recommended because the bottles do not have child-resistant caps.
The symptoms of belladonna toxicity may include:
- Seizures
- Trouble breathing
- Lethargy
- Excessive sleepiness
- Muscle weakness
- Skin flushing
- Constipation
- Difficulty urinating
- Agitation
The FDA also recommends against giving infants teething gels that contain the anesthetics lidocaine or benzocaine due to the risk of life-threatening side effects.
Do I have a Product Liability Lawsuit?
The Schmidt Firm, PLLC is currently accepting injury cases in all 50 states. If your infant had a seizure or belladonna toxicity from teething gel, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Product Liability Litigation Group or call toll free 24 hours a day at (866) 920-0753.
Attention Lawyers: We consider a referral from another law firm to be one of the greatest compliments. If your firm is interested in referring us a case or for us to send you a list of previous award judgments and/or average referral fees, please visit the Lawyer Referral section of our website.

 The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.
The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.

