Hundreds of women with breast implants have developed a dangerous cancer called Anaplastic Large Cell Lymphoma (ALCL), and at least 36 women have died of this disease. In the 1990s, $4 billion was paid in a massive class action settlement to 450,000 women who got sick from silicone breast implants.
What You Can Do & How We Can Help
The Schmidt Firm, PLLC is currently accepting breast implant induced injury cases in all 50 states. If you or a loved one has been diagnosed with cancer or had a doctor suggest breast implant removal because of a predisposition of cancer, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call toll free 24 hours a day at (866) 920-0753.
FDA Finds 733 Cases of Breast Implant Cancer, 36 Deaths Worldwide
As of January 2020, the FDA has found 733 cases of breast implant cancer and 36 deaths globally from Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Of these cases, 620 cases were in women with Allergan’s breast implants.
Allergan is Trying to Find 52,000 Women With Recalled Breast Implant
In June 2020, Allergan launched a campaign to try to track down 52,000 women with recalled Biocell® breast implants and warn them about their potential risk of cancer. The company has offered to pay $7,500 for out-of-pocket surgery costs for women with ALCL, and $1,000 for diagnostic testing for anyone else with the implants.
Cancer Victims File Class Action Lawsuits
In September 2019, another class action lawsuit was filed against Allergan by a woman from Illinois who claims the company knew about the risk of cancer from its textured breast implants for several years before recalling the implants in July 2019.
In August 2019, two women with cancer from their breast implants filed a class action lawsuit against Allergan. Both women say they were forced to pay out-of-pocket for surgery to remove and replace their recalled Biocell® breast implantst
Allergan Recalls Breast Implants for Cancer Risk
In a major reversal, the FDA has asked Allergan to recall BIOCELL® textured breast implants in the U.S. after finding 24 more deaths and a 6-fold increased risk of ALCL.
The recall comes 2 months after Allergan recalled the implants in Canada and 7 months after Allergan recalled the implants in Europe.
Last year, the FDA refused to ban Allergan’s textured breast implants because the agency did not have enough data. Now, the FDA says it has identified dozens of deaths and hundreds of known cases of breast implant-associated ALCL — including at least 620 cases of ALCL in women with Allergan’s recalled breast implants.
Allergan will stop selling the following textured breast implants:
- Textured Natrelle Inspira Breast Implants
- Natrelle 410 Breast Implants
- BRST Breast Implants
- Allergan Tissue Expanders 133Plus
Textured Breast Implants Linked to 400X Risk of Cancer
January 11, 2018 — A new study has found that silicone breast implants with a textured surface are 400-times more likely to cause a rare type of cancer compared to silicone breast implants with a smooth surface. Click here to read more.
Breast Cancer Survivors at Risk of Lymphoma
Breast cancer survivors often undergo a double mastectomy to reduce the risk of their cancer returning. When implants are used in reconstructive surgery, there is a low risk of developing lymphoma as a result of the implants. One breast cancer survivor from Missouri said:
“My whole world came crumbling down again. I had spent the past six years going to the oncologist every three months trying to keep cancer away, and here was something I had put in my body to try to help me feel more like a woman, and it gave me cancer.”
Breast Implants Linked to 500+ Reports of Lymphoma, 33 Deaths
In March 2017, the FDA confirmed that breast implants are associated with Anaplastic Large-Cell Lymphoma (ALCL), a rare but fast-growing type of non-Hodgkin lymphoma. As of July 2019, the FDA received over 500 reports of ALCL in women with breast implants, including 33 women who died. Textured implants have the highest risk of ALCL.
Problems With Textured Breast Implants
Recent studies have found that bacterial coatings (biofilm) on textured breast implants may trigger low-level infections, inflammation, and seroma (fluid buildup around the implant). The symptoms of ALCL may include: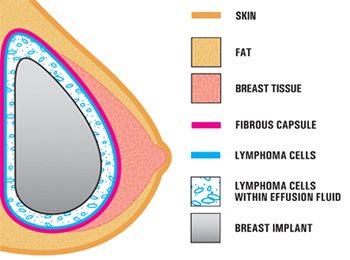
- Breast pain
- Swelling or lumps
- Asymmetry of the breast
- Fever
- Loss of appetite
- Tiredness
- Night sweats
- Weight loss
- Painless swelling in the armpit, neck or groin
- Enlarged lymph nodes
MemoryGel Breast Implant Lawsuit
In January 2017, a lawsuit was filed in Los Angeles by a woman who suffered health problems when her Mentor MemoryGel breast implants leaked silicone in her body. Her lawyer told Bloomberg, “We believe the problems with Mentor’s silicone implant are pervasive and may have harmed thousands of women. This suit may be just the tip of the iceberg.”
Mentor MemoryGel Lawsuit Filed in California
In September 2016, another lawsuit (PDF) was filed by a woman from Seattle who experienced fatigue, nausea, skin rashes, heavy metal poisoning, and other health problems that she blames on “systemic toxicity due to her body’s reaction to the toxic elements contained within the gel of the implants.”
$4 Billion Breast Implant Class Action Settlement
Breast implant lawsuits are nothing new. Around 450,000 women who were injured filed lawsuits and joined class action lawsuits in the 1980s and 1990s after studies found evidence of tissue disease. After several multi-million dollar jury awards, Dow Corning Corporation offered a $4 billion settlement and went bankrupt in 1995.
Breast Implant Lawsuit Settlement Amounts
In 1998, $3.2 billion in lawsuit settlements were paid to thousands of women who were injured by silicone breast implants. The payouts averaged $26,000 (or roughly $20,000 for a ruptured implant and $5,000 for surgery) plus between $10,000 and $250,000 for diseases.
FDA Lifts 14-Year Ban on Silicone Breast Implants
The FDA banned silicone breast implants in 1992 due to concerns about side effects and a lack of long-term safety data. In 2006, under pressure from the industry, the FDA agreed to lift the 14-year ban — but required manufacturers to complete several massive studies.
What Happened With the Safety Studies?
Mentor Worldwide became the first company allowed to sell silicone breast implants 2006. In exchange, Mentor was required by the FDA to enroll 80,000 women in 6 long-term safety studies lasting 10 years.
Only 3 years into the 10-year study, Mentor “lost track” of 79% of women who signed up when the study began. As a result of the massive dropout rate, the entire study was essentially meaningless.
Other manufacturers had similar problems. Allergan “lost track” of 40% of women with silicone breast implants just 2 years into a 10-year safety study. Low participation makes the studies completely useless for detecting rare side effects like cancer.
Studies do not show evidence that silicone gel-filled breast implants cause connective tissue disease, reproductive problems, or breast cancer. Low follow-up rates and other study limitations may limit interpretation of the data and preclude the detection of very rare complications.
What are Silicone Breast Implants?
Silicone gel-filled breast implants have an outer shell of silicone that is filled with silicone gel. They may contain toxic chemicals or heavy metals left over from the manufacturing process. This is also true for “cohesive gel” or “gummy bear” silicone implants that have recently become popular because they are advertised as leak-resistant.
List of Silicone Breast Implants
- Allergan Natrelle
- Allergan Natrelle Cohesive Silicone Gel Implants
- Mentor MemoryGel
- Mentor MemoryShape
- Sientra Silicone Gel Breast Implants
“Ticking Time Bomb” in Millions of Women
Breast implants are advertised as safe and permanent — but the reality is they are NOT lifetime devices. Nearly all of them will eventually rupture and leak. Even intact implants commonly “sweat” out silicon or platinum through the shell (called “gel bleed”). Breast implants can also pop open or break suddenly without causing any symptoms. This is called a silent rupture and it is why the FDA recommends MRI screening at 3 years post-implant and every 2 years afterward.
Symptoms of Leaking Breast Implants
- Decrease in breast size
- Change in breast shape
- Hard lumps over the implant or chest
- Pain or tenderness
- Tingling
- Swelling
- Numbness
- Burning sensation
What Happens When Breast Implants Leak?
Silicone is thicker than saline (salt-water), so when a silicone gel-filled breast implant ruptures, the gel may stay in the shell or the capsule of scar-tissue surrounding the implant. This is the best-case scenario.
The worst-case scenario — and far more common — is that silicone leaks outside the capsule surrounding the implant and is absorbed by the body. The leaked silicone may travel (migrate) away from the breast and create lumps in the lymph nodes, chest wall, or armpit. It is usually impossible to remove all silicone gel in other parts of the body.
What is Silicone Sickness?
“Silicone sickness” is a whole-body illness that occurs when toxins trigger an immune response. Leaking breast implants are known to cause tissue inflammation and foreign body reactions when the body rejects the implant. Silicone leaking out of breast implants might also cause life-threatening tissue diseases like scleroderma or systemic sclerosis — but without adequate safety studies, no one knows for sure.
What is the Risk?
Dr. Edward Melmed, a plastic surgeon in Dallas, Texas, spoke at a 2-day meeting when the FDA was considering recalling all silicone breast implants due to a lack of long-term safety data in 2011. He called silicone an “industrial toxin” and also warned that 50% of silicone breast implants rupture by 10 years, 72% by 15 years, and 92% by 20 years.
Signs & Symptoms of Silicone Sickness
- Fatigue and weakness
- Breast pain
- Connective Tissue Disease (CTD)
- Lupus
- Joint or muscle pain
- Rheumatoid arthritis
- Cognitive dysfunction (brain fog, memory loss)
- Toxic Shock Syndrome
- Fibromyalgia diagnosis
- Systemic sclerosis
- Non-Hodgkin’s Lymphoma
- Anaplastic Large Cell Lymphoma (ALCL)
- Swollen lymph nodes
- Skin rash or hives
- And more
New Wave of Breast Implant Lawsuits?
Breast implants are Class III (“high risk”) medical devices that must go through a rigorous protocol called Premarket Approval (PMA) before they are allowed on the market. In 2008, the U.S. Supreme Court ruled in Rigel vs. Medtronic that manufacturers of PMA-cleared medical devices are immune from lawsuits involving injuries.
That means breast implant injury lawsuits would normally face an impossible legal battle. However, there are exceptions — such as when a manufacturer violates requirements of PMA. In the case of silicone gel-filled breast implants, a new wave of lawsuits accuse manufacturers of inadequately testing the implants for safety and failing to warn about side effects.
Do I have a Breast Implant Lawsuit?
The Schmidt Firm, PLLC is currently accepting breast implant induced injury cases in all 50 states. If you or a loved one has been diagnosed with cancer or had a doctor suggest breast implant removal because of a predisposition of cancer, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call toll free 24 hours a day at (866) 920-0753.
Attention Lawyers: We consider a referral from another law firm to be one of the greatest compliments. If your firm is interested in referring us a case or for us to send you a list of previous award judgments and/or average referral fees, please visit the Lawyer Referral section of our website.


 The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.
The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.

