The FDA has confirmed 36 deaths and over 700 cases of a rare cancer that is caused by breast implants. Cancer is more likely in women with textured implants. After 84% of cases were linked to Allergan’s Biocell® implants, a recall was issued in July 2019.
What You Can Do & How We Can Help
The Schmidt Firm, PLLC is currently accepting breast implant induced injury cases in all 50 states. If you or somebody you know has been diagnosed with Anaplastic Large Cell Lymphoma (ALCL), you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call toll free 24 hours a day at (866) 920-0753.
UPDATE: FDA Reports 733 Cases of Breast Implant Cancer, 36 Deaths Worldwide
As of January 2020, the FDA has identified a total of 733 cases of breast implant cancer and 36 deaths worldwide. All of the women developed Anaplastic Large Cell Lymphoma (BIA-ALCL). Of the 733 cases, 620 were women with Allergan breast implants.
Do Breast Implants Cause Cancer?
Breast implants are associated with Anaplastic Large Cell Lymphoma (ALCL), a rare but aggressive type of non-Hodgkin’s lymphoma. It is a cancer of the immune system that starts in white blood cells.
Breast Implant Recall
In 2019, Allergan recalled breast implants in the U.S. after they were linked to a 6-fold increased risk of cancer compared to similar implants. At least 12 deaths and 481 cases of a cancer called Anaplastic Large Cell Lymphoma (ALCL) have been linked to Allergan’s recalled implants.
Is ALCL Breast Cancer?
ALCL in the breast is not like other types of breast cancer. It does not show up on a mammogram. ALCL starts in blood cells called T-lymphocytes (T-cells) that fight “foreign invaders” in the body.
What is the Problem?
When you get breast implants, scar-tissue creates a capsule around the implant. Some women never fully heal. Instead, they develop low-level inflammation or an infection. T-cells multiply to fight this infection.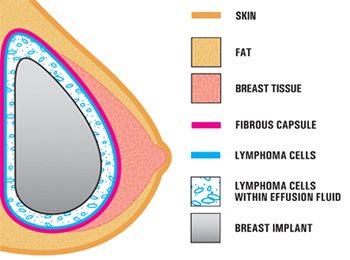
Do I Have ALCL?
Over time, the risk increases of a cancerous mutation that causes T-cells to multiply uncontrollably. The symptoms of this complication are usually related to a buildup of fluid around the implant, called a seroma. Most women with ALCL are diagnosed when a doctor removes a sample of this fluid and finds cancerous T-cells.
Symptoms of ALCL Breast Cancer
ALCL grows fast but it does not cause severe symptoms right away. In women with breast implants, most cases are only diagnosed when the implants are removed due to pain, asymmetry, lumps, or swelling.
Other symptoms of lymphoma may include:
- Fever
- Loss of appetite
- Tiredness
- Night sweats
- Weight loss
- Painless swelling in the armpit, neck or groin
- Enlarged lymph nodes
What is the Risk?
The risk is very low, but ALCL is more likely in women with breast implants. Textured implants have the highest risk. Several hundred cases have been reported out of millions of women with breast implants, but it is likely that more cases of ALCL were never reported.
In the general population, ALCL occurs in approximately 1 in 500,000 women. ALCL in the breast is even more rare, with only 3 in 100 million cases diagnosed each year. Breast implants increase the risk.
FDA Reports 33 Deaths and Over 573 Cases of ALCL
As of 2019, the FDA has found 573 cases of breast implant-associated ALCL, including 33 deaths. Breast implants are specifically linked to ALK-negative ALCL, which is the most dangerous type because it often relapses after treatment. According to the FDA:
“Most cases of breast implant-associated ALCL are treated by removal of the implant and the capsule surrounding the implant and some cases have been treated by chemotherapy and radiation.”
Textured vs. Smooth Breast Implants
ALCL is significantly more common in women with textured breast implants vs. smooth implants. All breast implants have an outer shell that is made of silicone rubber. The shell is filled with either saline (salt water) or silicone gel. Textured breast implants have a shell with a rough surface like soft sandpaper. Smooth breast implants have a shell with a slippery or shiny surface.
Bacterial Contamination on Breast Implants
Bacterial contamination is nearly impossible to avoid when a doctor is squeezing a breast implant into the body during surgery. The immune system can easily kill bacteria on a smooth-surface breast implant, but on a textured implant, bacteria can hide and create a coating (biofilm).
Recent studies show massive increases in biofilm and bacterial contamination on textured breast implants vs. smooth implants. This increases the risk of inflammation, scar-tissue, and a complication called contracture that occurs when scar-tissue hardens.
Chronic inflammation is also a risk-factor for lymphoma, which might help explain why women with textured breast implants seem to have a higher risk of ALCL. Experts are still investigating this risk to better understand the link between silicone breast implants and cancer.
Cancer Risk of Saline vs. Silicone Gel-Filled Implants
The number of cases of ALCL has skyrocketed in recent years — and the popularity of silicone breast implants with textured surfaces may explain why. The statistics are stunning.
Only 17 cases of ALCL in women with breast implants were reported to the FDA from January 1997 through May 2010. For most of this time, only saline breast implants were on the market in the U.S.
The FDA un-banned silicone breast implants in 2006. They rapidly grew in popularity and are now significantly more popular than saline implants. From August 2010 to September 2015, the FDA received over 250 reports of ALCL in women with breast implants. Another 100 cases were reported by February 2017.
As of February 2017, the FDA has received 359 reports of ALCL in women with breast implants. Out of 312 reports with information on the type of implant, 186 were silicone and 126 were saline. The numbers are similar, but the risk is not. Silicone implants were only sold for 11 years and saline implants were never banned.
Safety Studies Worthless for Investigating Cancer
The FDA banned silicone gel-filled breast implants in 1992 due to safety concerns and over 450,000 injury lawsuits. The FDA lifted the ban in 2006 after manufacturers promised to conduct 10-year safety studies, but manufacturers “lost track” of so many women that the studies were worthless for investigating cancer.
Do I have a Breast Implant Lawsuit?
The Schmidt Firm, PLLC is currently accepting breast implant induced injury cases in all 50 states. If you or somebody you know has been diagnosed with Anaplastic Large Cell Lymphoma (ALCL), you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call toll free 24 hours a day at (866) 920-0753.
Attention Lawyers: We consider a referral from another law firm to be one of the greatest compliments. If your firm is interested in referring us a case or for us to send you a list of previous award judgments and/or average referral fees, please visit the Lawyer Referral section of our website.


 The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.
The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.

