Lawsuits have been filed by people who were injured by a Bard PowerPort.
What You Can Do & How We Can Help
The Schmidt Firm, PLLC is currently accepting Bard PowerPort induced injury cases in all 50 states. If you or somebody you know was injured by a Bard PowerPort, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call toll free 24 hours a day at (866) 920-0753.
UPDATE: Bard PowerPort Lawsuits Centralized in MDL
Hundreds of Bard PowerPort Lawsuits have been filed by people who were injured. These lawsuits are centralized in the Bard PowerPort MDL No. 3081, which is located in the U.S. District Court for the District of Arizona under the Honorable David G. Campbell.
Bard PowerPort Lawsuit Claims It Leaked Chemo Drugs
In July 2023, a lawsuit was filed by a terminally-ill man with colon cancer who claims that his Bard PowerPort leaked chemotherapy medication directly into his body. Click here to read more.
300,000 Bard PowerPorts Implanted Worldwide
The Bard PowerPort is one of the most popular ports for people with cancer and other long-term medical conditions that require routine IV access. Approximately 300,000 PowerPort devices have been implanted in patients worldwide. Unfortunately, some of them have experienced side effects.
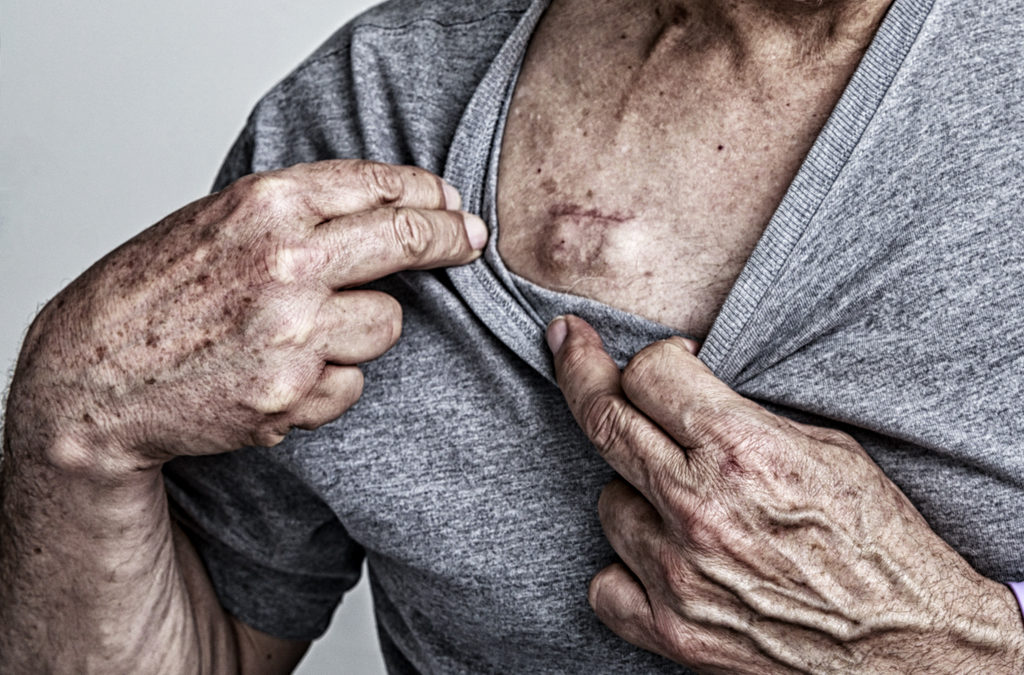
What is the Bard Power Port?
The Bard PowerPort is a medical device that is also called a Port-a-cath or a chemotherapy port. The port is implanted under the skin and connected to a catheter tube that empties into a vein.
The Bard PowerPort consists of two main parts:
- The port, which is a soft silicone device that is implanted totally under the patient’s skin on their arm or chest. The port is where a needle is inserted.
- The venous catheter, which is a long flexible tube that connects the port to a large central vein deep inside the body.
What Patients Need an Implanted Port?
The Bard PowerPort and similar devices are typically implanted in patients who need repeated IV medications, fluids, or blood draws for conditions like cancer, Crohn’s disease, severe infections, kidney failure, IBD, and more.
The port gives nurses and doctors an easy access point to deliver medications, IV fluids, nutrition, blood products, or to draw blood samples.
The catheter tube is also connected to a large vein deeper inside the body, rather than a small vein near the surface of the skin that can be easily damaged by repeated IVs and blood draws. This helps avoid “chemo veins” and other vein injuries.
What is the Problem?
Hundreds of lawsuits have been filed by people who were injured by the Bard PowerPort. According to federal judges:
“Defendants manufacture the catheter component of their port devices with a concentration of barium sulfate that is too high, which reduces the material integrity of the catheter, and can lead to injuries, including infection, fracture of the catheter, migration of the catheter, and thrombosis.”
Catheter Fracture
One of the most serious problems associated with implanted ports occurs when the catheter fractures, cracks, breaks, or leaks medications. In some cases, broken pieces of the catheter travel in the patient’s bloodstream, where they can damage veins or internal organs.
Catheter Infection
Infections are a common problem associated with implantable ports, usually soon after the device is implanted. The risk includes bloodstream infections (sepsis), tissue necrosis around the port, and chronic pain or soreness around the port.
Catheter Migration & Breakage
Catheter migration is a problem that occurs when the tube detaches from the port and moves away from its intended location. Sometimes, broken parts of the catheter may even travel in the patient’s bloodstream, get stuck in the heart, or cause other life-threatening complications. Emergency surgery is usually necessary to remove the broken pieces when a port catheter migrates or breaks inside the body.
Bard PowerPort Lawsuit Filed by Injured Woman
In February 2023, a lawsuit was filed by a woman from Missouri who developed blood clots and had to have her Bard PowerPort removed only 3 weeks after it was implanted.
The plaintiff, Patrice T., was implanted with a Bard PowerPort Single Lumen ClearVue Implantable Port in March 2022 to make it easier to receive chemotherapy for her colon cancer. Unfortunately, only about 3 weeks after the PowerPort was implanted, she was hospitalized with blood clots (also known as a “thromboembolism”).
The lawsuit was filed February 10, 2023 in the U.S. District Court for the Western District of Missouri — Case Number 4:23-cv-00100-BP.
Bard PowerPort Recall (2021)
In March 2021, Bard initiated a Class II recall for certain PowerPort Duo M.R.I. Implantable Ports. According to the recall, “catheters may experience difficulty in flushing, infusion, and/or aspiration, and septum dislodgements.”
Do I have a Bard PowerPort Lawsuit?
The Schmidt Firm, PLLC is currently accepting Bard PowerPort induced injury cases in all 50 states. If you or somebody you know was injured by a Bard PowerPort, you should contact our lawyers immediately for a free case consultation. Please use the form below to contact our Defective Medical Device Litigation Group or call toll free 24 hours a day at (866) 920-0753.
Attention Lawyers: We consider a referral from another law firm to be one of the greatest compliments. If your firm is interested in referring us a case or for us to send you a list of previous award judgments and/or average referral fees, please visit the Lawyer Referral section of our website.

 The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.
The Schmidt Firm, PLLC has been recognized as one of the nation’s leading plaintiffs' law firms and handles cases in all 50 states. We are very proud of our legal achievements, but equally self-respecting of our firm's reputation for providing personal attention to each and every client we represent.

